
|

|
Iron Production in the Viking Age
 |
Although Norse people knew of mining and mined some iron ore in a variety of locations throughout Scandinavia, most Viking era iron was smelted from bog iron. The photo to the left shows the bog at Rauðanes in Iceland, where Skallagrímur Kveldúlfsson, one of the early settlers in Iceland, had his smithy. Chapter 30 of Egils saga describes him as a "great smith" who "smelted a lot of bog iron in winter". |
 |
The saga says that Skallagrímur couldn't find an anvil stone to suit him, so he rowed out into Borgarfjörður one evening in his boat. He dove to the bottom and brought up a suitable boulder for his anvil stone. The anvil stone, with its hammer marks, still stands at Rauðanes (left), but it has been moved away from the shore. Ruins (right), which are the probable site of Skallagrím's smithy, are located closer to the shore. |
 |
|
At the Norse settlement in L'Anse aux Meadows in Newfoundland, Canada, there is clear evidence that Norsemen harvested and smelted bog iron to use as the raw material for the iron rivets they fabricated to repair their ships there 1000 years ago. Where streams run from nearby mountains through a peat bog, bog iron can almost always be found. That combination was probably visible to Norse explorers even from on board their ships in the cove at L'Anse aux Meadows. It occurs in glaciated regions throughout the world, and so would have been very familiar to the Norse explorers at Vínland. |
 |
 |
Streams carry dissolved iron from nearby mountains. In the bog, the iron is concentrated by two processes. The bog environment is acidic, with a low concentration of dissolved oxygen. In the acidic environment of the bog, a chemical reaction forms insoluble iron compounds which precipitate out. But more importantly, anaerobic bacteria (Gallionella and Leptothrix) growing under the surface of the bog concentrate the iron as part of their life processes. Their presence can be detected on the surface by the iridescent oily film they leave on the water (left), another sure sign of bog iron. In Iceland (right), the film is called jarnbrák (iron slick). |
 |
|
When a layer of peat in the bog is cut and pulled back using turf knives (right), pea sized nodules of bog iron can be found and harvested. Although the iron nodules are reasonably pure, there aren't many of them. They are, however, a renewable resource. About once each generation, the same bog can be re-harvested. |
 |
 |
In some regions (particularly Sweden), iron ore, rather than bog iron, was the raw material for smelting. The ore was in the form of "red earth" (rauði), a powdery ocher. Regardless of the source, the raw materials were usually roasted to drive off moisture before being smelted. The roasting also served to "crack" the surface of the iron ore, making it more porous so that the gases in the smelting furnace could enter and react with the iron more easily. |
| The iron ore shown to the right was excavated from Skógar, a
Viking-age iron-making site in Iceland. The larger piece is about 2cm
(less than 1in) in the long dimension. Sometime about 1000 years ago,
someone gathered this iron ore, processed it, transported it to the iron
smelting site, but for unknown reasons, never used it in a smelt. Once the dry nodules or ore was in hand, the lengthy process of smelting the iron began. |
 |
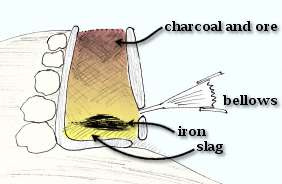 |
The smelting operation was performed in a bloomery furnace, a small clay or clay-lined shaft shown schematically to the left. The furnaces were roughly circular, and about 80cm (32 in) tall and about 30cm (12in) in diameter. A modern replica is shown to the right. |
 |
 |
The clay was actually a homogenous mixture of sand, fiber, clay, and water (left). The fiber was introduced into the mix as horse dung, in which the undigested hay provided the fiber. This mix had the needed structural properties to contain the smelting materials, as well as appropriate insulating properties. The walls must radiate enough heat to keep the inside surface from melting into the slag during the smelt. |
 |
 |
The furnace could be built around a tapered wooden form, or simply by building it up from an earthen base (left), tying off the outside with rope to prevent sagging, and filling the inside with a sand/ash mixture to draw water out of the clay. As the clay dried, the wooden form or the sand/ash mixture was removed, and the drying was completed with a gentle wood fire inside the furnace (right). |
 |
 |
Recent (and on-going) archaeological research suggests that smelting furnaces in Iceland were made from stacked turf, with a combustion shaft in the center, lined with a thin layer of clay. We at Hurstwic have run this design several times (left) and find it the smoothest running of any of the designs we have tried. A slide show which describes this furnace constuction and operation in much more detail is available here. |
|
To begin the smelting operation, a fire was started inside the furnace with a natural draft, to prevent the thermal shock from rapid heating from damaging the furnace. One warmed, an air blast was supplied through the side of the furnace with a bellows, and the bore of the furnace was filled with charcoal. The photo to the right shows a modern replica furnace at the start of this process. Air was forced into the furnace through the tuyere, seen on the left side of the furnace. Archaeological evidence suggest that Viking-age furnaces used a tuyere made of copper. Our replica furnace used a modern (and affordable) ceramic tuyere. The placement of the tuyere seems to be a critical parameter in the success of these replica furnaces. This replica used air delivered by an electrically powered air pump. The air was adjusted to control the burn rate, and ore and charcoal were regularly added to the top of the furnace in roughly a 1:1 ratio (by weight). Inside the furnace, the temperature reached 1100-1300ºC (2000-2400ºF) at the bottom of the furnace near the iron. A reducing atmosphere was created, rich in carbon monoxide. The gas scavenged the oxygen from the iron compounds in the ore, converting them to elemental iron:
Not surprisingly, the reactions are considerably more complex than this simple equation would indicate. The temperature in the furnace ranged from 300ºC near the top to as high as 1300ºC near the bottom. Different chemical reactions occurred in the different temperature zones of the furnace. |
|
 |
Throughout the smelting process, additional fuel (left) and ore (right) were added at the top of the furnace. The process was tended constantly, adjusting the fuel, ore, and air to optimize the results. The smelting operation lasted for many hours. |
 |
 |
Slag (consisting of oxides of iron, silicon, and other elements) was
both the waste product of the smelting process, and an essential element
to the smelting process. Liquid slag at the bottom of the furnace was
contained in a bowl formed by solidified slag. Elemental iron formed in
the upper part of the furnace dropped down and collected in the slag
bowl.
If the liquid slag rose to a high enough level to block the air from the tuyere, the iron making process could be impacted. The level of the slag (and the falling of the iron) could be monitored, both by the sound of the air passing through the tuyere, and by visually watching the process through the bore of the tuyere from the outside end. If the slag level rose too high, the tap arch, a door in the bottom of the furnace, could be opened and liquid slag drained (left). The liquid slag flows like water. Solidified slag (right) from archaeological sites provides a great deal of information about the smelting process in the Viking age. |
 |
 |
The process continued for a very long time (many hours over the better part of a day) and required constant attending to maintain optimal conditions inside the furnace. Fuel, air, ore, and slag required constant attention. When completed, a section of the lower furnace was removed (right), and a frothy iron sponge-like material called a bloom (blástrjárn) was levered free of the furnace walls. |
 |
|
|
The bloom (left, when hot, and right, when cold) was a mixture of low-carbon iron, slag, and charcoal. The surrounding slag and charcoal were knocked off. |
 |
 |
Immediately after removing from the furnace, the remaining material was compacted with sledges to consolidate the material. This work is best done while the bloom retains the heat from the smelting process. A wooden log anvil (left) is often used, since the heat of the bloom burns a depression in the wood, helping to hold the bloom in position as it is worked. The bloom was refined by folding it, which mechanically served to homogenize the material and to drive out the impurities, such as slag. The folding process was repeated multiple times to create cleaner, more highly-refined material. The desired end result was a billet of malleable low-carbon iron, ready to be forged to fabricate the required articles, or to be turned into currency bars for trade, discussed below. |
In the past, I believed that the quality of the iron obtained was highly variable because the smelting process was difficult to control. Yet modern smiths using replica Viking-age bloomery furnaces are routinely turning out high quality iron, which suggests they have good control of the process. Regardless, the process was inefficient; a lot of iron was left in the slag. Modern practitioners doing bloomery smelts do not think this is a bad thing: low levels of iron in the slag seem to predict poor quality iron in the bloom.
We have analyzed two different blooms that we created and compared them to a Viking-age iron bloom. The results of that comparison is here.
|
Despite the difficulty in smelting iron in the Viking age, evidence suggests there were places that smelted prodigious quantities of iron in this era. At Eiðar in east Iceland (right), the slag heaps reach the height of the two-story buildings behind them. Analysis of the slag suggests that something like 1000 tonnes of iron was created here in a couple of centuries after the settlement of Iceland, laboriously smelted from bog iron collected in the district. The evidence of the slag is hard to refute, and only serves to generate many more questions. It represents, on average, one smelt every day for several centuries! Who did all this work? How was the iron distributed and in what form and to whom? How many furnaces were needed, and where did the charcoal come from in order to fuel these furnaces? Where did the ore come from and how was it collected? |
 |
The labor and skill required to make an iron bloom meant that they were valuable commodities. The distribution of iron finds at the Viking-age house at Hólmr in Iceland suggests that iron blooms were used as cult offerings there.
|
At L'Anse aux Meadows, the iron was probably used to make rivets and washers for ship repair. The wrought iron was rich in silicate impurities, which formed a glassy surface on the iron. This is visible on the parts even today (right). The surface helped protect the iron from rust, even when immersed in sea water. |
 |

|
Most domestically produced iron in the Norse era was tediously produced from bog iron. Because of the time consuming processes used to create it, smelted iron was valuable. Roughly worked iron bars (currency bars) were used as trade goods (left). These bars are from Norway and are about 30cm (12 inches) long. It has been suggested that the shape of the currency bars indicated the quality of the iron. |
|
The axe head blanks shown to the right were also trade goods. They were probably exported from Norway and were on their way to a trading center in Denmark when they were lost. They are about 20cm (8inches) long and were found threaded on a wooden stave for convenient handling during shipping. |
 |
 |
Because of their expense, iron tools and weapons were highly valued. The loss of an iron tool from a Norse farmstead was a disaster, especially if it were a major tool like an axe or scythe. A typical farm in the Viking age probably owned no more than 40-50kg (100lbs) of iron, in the form of tools, weapons, and cooking equipment. The Mästermyr chest (a reproduction is shown to the left) is an oak tool chest filled with tools that belonged to a master smith in the 11th century. The chest and its contents were found in Mästermyr, Sweden in 1936. The chest contained an assortment of tools for working wood and metals (including hammers, axes, adzes, saws, spoon-borers, tongs, shears, files, and others), along with raw materials and finished products. It's been suggested that its owner lost the chest while crossing the mýrr (bog) that gives Mästermyr its name. The loss must have been a devastating blow to its owner. |
|
Viking-age smithies tended to be smaller than what we might imagine today: smaller physical spaces; smaller forges; smaller anvils; smaller fires; smaller sets of tools. Many of the tools would not look out of place in a modern forge, but due to the cost of making iron, less iron was used. The hammer was probably under 1kg (2lbs), the anvil perhaps 5kg (11lbs) with a work surface about 10cm square (4in square). The charcoal fired forge, likewise, was probably capable of heating only 10cm of the workpiece at a time. The small size of typical smithies is confirmed from archaeological vidence. The ruins of the smithy at Stöng in south Iceland is shown to the right, with the foundation stones around the periphery, and a stone cistern in the center to hold water for cooling the workpiece. |
 |
 |
When the old church at Reykholt in west Iceland (shown to the left) was renovated, the ruins of a Viking-age smithy were found under the church. The smithy was 2m x 3m (about 6.5ft x 10ft). There was a stone-lined cistern in the center of the floor, presumably for holding water. Next to the cistern was the hearth, about 80cm (31in) in diameter filled with charcoal and soot. A stone lined channel ran in the floor from the hearth to the outside and is thought to be an air channel for the fire. It is thought that even in a fire this small, it was possible to heat treat large items by continually moving the workpiece in the fire. |
|
The smithy at Vatnsfjörður (foreground in the photo to the right) in west Iceland had a central hearth, and a depression along the wall that held substantial amounts of iron slag. Here, as in other Viking-age smithies, it seems likely that the same smith smelted the iron and then fabricated useful articles from it. The longhouse ruins with its central firepit can be seen in the distance behind the smithy. |
 |
The interpretation of Viking-age smithies remains troublesome. For example, the smithy at Reykholt has a rectangular depression in the floor adjacent to the cistern and the hearth which has been interpreted as the place where the smith put his feet as he sat and worked. At least some modern smiths I've talked to don't find this interpretation convincing.
 |
Surviving anvil stones are quite low to the ground, too low for comfortably working on a piece. The anvil stone at Aðalból in East Iceland is shown to the left. On the other hand. the wood carving from a 12th century church in Norway (shown to the right) illustrates a scene from a Norse heroic myth in which Reginn reforges a sword for his foster brother Sigurðr. As depicted in this carving, the anvil seems uncomfortably low to the ground. On the third hand, smiths in various world cultures are known to have worked: standing; sitting; kneeling; and hunkering down over the anvil, as Reginn appears to be doing in the carving. So perhaps Viking-age smiths were comfortable working in positions that a modern smith might find awkward. The carving also shows some details of the bellows used in the Viking era. Two bellows worked alternately blew into the fire through a soapstone bellows shield, which kept the heat of the fire from reaching the wood and leather bellows. |
 |
 |
This soapstone artifact was found in Denmark and is interpreted as a bellows shield with a depiction of Loki with his lips sewn shut, the result of his loosing his wager with the dwarf smiths Brokkr and Sindri, told in the myths. The stone is about 20cm (8in) across, with a hole near the base presumably to allow air from the bellows to reach the fire. |
|
It seems likely that iron arms and armor were treasured and passed from generation to generation. Lifetimes of hundreds of years seem possible for some iron articles such as helmets. When one compares the probable date of manufacture with the probable date of burial of some archaeological finds, one is forced to conclude that some iron items were used for a century or more before being buried with their final owner. |
 |
|
|
©1999-2026 William R. Short |
|
Hurstwic organized an intense research project in 2018-2019 to understand how iron smelting was performed in Viking-age Iceland. For several reasons, it was clear that it differed from iron-making in Viking-age Scandinavia, but the details of how it was accomplished were lost. Hurstwic gathered a team of experts from Iceland, Europe, and North America. Together, we rediscovered the lost secrets of iron-making in Viking-age Iceland. In a public festival at the the Viking house Eiríksstaðir, we made the first bloomery iron in over 700 years made from Icelandic materials and furnaces. Information about our festival is on the festival page. A picture album of our activities is here, and a video clip about the festival that aired on Landinn, an Icelandic television show, is here. The finished iron seen in the photo (right) was donated to Þjóðminjasafn Íslands (National Museum of Iceland) and was a part of a special exhibit on Viking-age Iron Smelting in Iceland, based on Hurstwic's research. A video guided tour of the exhibit can be seen here on YouTube. Hurstwic published a final report on our experiments in Viking-age iron smelting in Iceland. |
|
Hurstwic's William R. Short and Reynir A. Óskarson gave a lecture at the National Museum of Iceland on experimental archaeology, and our use of this research approach in our iron smelting work in Iceland. A video recording of the lecture can be seen here on YouTube. (The introduction is in Icelandic, and the lecture is in English.)
A video lecture on Hurstwic's iron smelting experiments presented by William Short on behalf of the Gene Winter Chapter of the Massachusetts Archaeological Society can be seen here on YouTube.